

Our group's interests are in the research areas shown below.
Copper(I) oxalate complexes
Copper(I) oxalate complexes are promising materials for the chemical vapor deposition of metallic copper, important in the semiconductor industry for the manufacture of high-speed integrated circuits, e.g., on computer chips. On heating, these complexes release their ligands, after which the bridging oxalate group decomposes cleanly to two molecules of carbon dioxide with the concomitant deposition of copper metal. Our lab is currently engaged in synthesizing and studying novel copper(I) oxalate complexes and related compounds. Structural characterization is carried out in collaboration with Prof. Arnold Rheingold's group at the University of California, San Diego and Dr. Robert Papoular, CEA-Saclay, France.
4_rev2.jpg)
2_rev2.jpg)
4oxH_rev2.jpg)
References
1. A. T. Royappa, A. D. Royappa, R. F. Moral, A. L. Rheingold, R. J. Papoular, D. M. Blum, T. Q. Duong, J. R. Stepherson, O. D. Vu, B. Chen, M. R. Suchomel, J. A. Golen, G. André, N. Kourkoumelis, A. D. Mercer, A. M. Pekarek, and D. C. Kelly, “Copper(I) oxalate complexes: Synthesis, structures and surprises,” Polyhedron 119, 563 (2016).2. A. T. Royappa, J. R. Stepherson, O. D. Vu, A. D. Royappa, C. L. Stern, and P. Müller, “Tetrakis(acetonitrile)copper(I) hydrogen oxalate-oxalic acid-acetonitrile (1/0.5/0.5),” Acta Crystallographica E69, m544 (2013).
3. A. D. Royappa, J. A. Golen, A. L. Rheingold, and A. T. Royappa, “µ-Oxalato-bis[bis(triphenylphosphine)copper(I)] dichloromethane disolvate,” Acta Crystallographica E69, m126 (2013).
Hyperbranched polymers
Our group's work in this area is focused on the synthesis and derivatization of new hyperbranched polymers. Our starting point is usually the cationic ring-opening polymerization of glycidol (1). This inexpensive, commercially available epoxide monomer can be polymerized to yield polyglycidol (2), a hyperbranched, water-soluble polyether-polyol with numerous terminal hydroxyl groups. Polyglycidol (PGly) may be considered a highly branched analog of polyethylene glycol (PEG), a well-known biocompatible polymer. Current research initiatives in the group center around creating derivatives (3) of polyglycidol (i) by copolymerization with other monomers and (ii) by reaction with small molecules of biological interest.
For example, PGly can easily be activated by reaction with standard sulfonyl chlorides such as tosyl chloride or tresyl chloride. The activated PGly reacts readily with, e.g., proteins, peptides and amino acids. We are currently examining such bioconjugates for enhanced immunological and bactericidal properties.
References
1. A. T. Royappa, M. R. Vashi, C. L. Russo, and A. C. Blackwell, “A comparison of the cationic ring-opening polymerizations of 3-oxetanol and glycidol,” Macromolecular Research 21, 1069 (2013).2. Y.-C. Huang, A. T. Royappa, S. Tundel, K. Tsukamoto, and V. Sharma, “Biocompatibility of Polyglycidol with Human Peripheral Blood Mononuclear Cells,” Journal of Applied Polymer Science 111, 2275 (2009).
3. A. T. Royappa and R. L. McDaniel, “Copolymerization of Glycidol with Functionalized Phenyl Glycidyl Ethers,” Journal of Applied Polymer Science 97, 1462 (2005).
4. A. T. Royappa, M. L. Vogt, and V. Sharma, “Composition and Long-term Stability of Polyglycidol Prepared by Cationic Ring-Opening Polymerization,” Journal of Applied Polymer Science 91, 1344 (2004).
5. A. T. Royappa, “Synthesis and Characterization of a Hyperbranched Copolymer,” Journal of Chemical Education 79, 81 (2002).
6. A. T. Royappa, N. Dalal, and M. W. Giese, “Amphiphilic Copolymers of Glycidol with Nonpolar Epoxide Comonomers,” Journal of Applied Polymer Science 82, 2290 (2001).
Multivariable fitting of chemical data
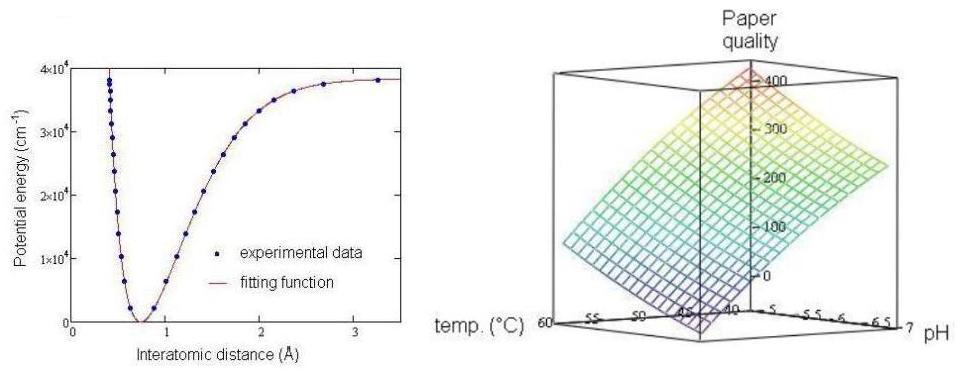
Multivariable analysis has long been generally accessible with the advent of built-in fitting routines in mathematical software packages. For example, Mathcad (Parametric Technology Corp.) contains the genfit routine, incorporating the Levenberg-Marquardt algorithm, for fitting multivariable data. This routine has been successfully usedby our group to fit data sets of chemical relevance, such as experimental diatomic interaction potential data (generally Rydberg-Klein-Rees, or RKR data) and process data on the dependence of paper quality on manufacturing variables in paper-making. Our group is currently at work creating novel, easy-to-use, closed-form mathematical functions capable of fitting experimental RKR data for a variety of diatomic molecules. Examples of our fitting results are shown below for the diatomic interaction potential of dihydrogen (left) and for paper quality vs. temperature and pH (right).
References
1. A. T. Royappa, V. Suri, S. E. Genet, and D. J. Pope, “Some New Closed-Form Empirical Modified Lennard-Jones Potentials,” Journal of Undergraduate Chemistry Research 9, 102 (2010).2. A. T. Royappa, V. Suri, and J. R. McDonough, “Comparison of Empirical Closed-Form Functions for Fitting Diatomic Interaction Potentials of Ground State First- and Second-Row Diatomics,” Journal of Molecular Structure 787, 209 (2006).
3. H. Zhang, A. F. Nitzman, and A. T. Royappa, “Statistical Modeling and Sizing Determination Guides for Dispersed Rosin Sizes,” TAPPI Journal 3, 3 (2004).
4. A. F. Nitzman and A. T. Royappa, “Sizing Variations of Dispersed Rosin Sizes with Fortification, Hardness, pH and Temperature,” TAPPI Journal 2, 8 (2003).